CHM580
SPECTROCHEMICAL METHODS OF ANALYSIS
EXPERIMENT 1:
FOURIER TRANSFORM INFRARED
SPECTROSCOPY- FTIR
ANALYSIS OF
ASPIRIN-PHENACETIN-CAFFEINE (APC) TABLET
ABSTRACT
This
experiment is done to identify the functional groups in IR spectra of standard
compound aspirin, phenacetin and caffeine, to identify the functional groups
present in aspirin-phenacetin-caffeine tablet, and to identify the functional
groups present in the unknown sample. The ASA, phenacetin, caffeine, aspirin,
polystyrene and unknown sample are analyzed by using IR spectroscopy in a form
of solid compound. KBr pellet is used and mix with each of the compound in
order to obtain the IR spectra of each of the compound. The unknown sample is
determined to be benzoic acid according to its IR spectra.
OBJECTIVE
To identify the functional groups in IR
spectra of standard compound aspirin, phenacetin and caffeine, to identify the
functional groups present in aspirin-phenacetin-caffeine tablet and to identify
the functional groups present in the unknown sample.
SAMPLE
-
Unknown sample
CHEMICALS
-
Aspirin-phenacetin-caffeine
tablet (APC tablet)
-
Acetylsalicyclic
acid (ASA)
-
Phenacetin
-
Caffeine
-
Aspirin
-
KBr powder
APPARATUS
-
Agate mortar and pestle
-
Spatula
-
Handpress
INSTRUMENT
Michelson
Interferometer
PROCEDURE
The
agate mortar and pestle was placed in the fume hood. Then, 1.0 mg of solid
sample was grinded into powder in agate mortar for 1 minute. Then, 80.0 mg of
KBr powder was added into the sample powder, and it was grinded again with
pestle for 30 seconds. After that, the mixture was scraped into the middle with
spatula, and it was grinded again for 15 seconds. Then, the mixture was heaped
in the center of the mortar.
One
fourth of the KBr mixture was transferred into the collar of the handpress.
Then, the anvil was placed with die pin. After that, the die set was lifted and
transferred to the unit. The dial pressure was rotated until the upper ram of
the handpress touched the upper anvil on the die assembled. Then, the mixture
was slowly compressed for 2 minutes. Then, the unit was tilted back and the
handle was opened. The die set was removed from the unit. Then the pellet was
weighed. After that, the collar contained the KBr pellet was placed onto the
sample holder in the sample compartment. Then, IR spectrum was obtained. After
that, KBr pellet was removed from the collar and the pellet material was placed
into the contained labeled “Recover KBr Pellets”. Then, the metal apparatus was
washed with water and dried in the oven.
RESULTS
Table 1.1: The table of IR frequencies
of the ASA from IR spectrum.
Frequency range (cm-1)
|
Frequency (cm-1)
|
Type of bond
|
Compound type
|
3300-2500
|
2917.28
|
O-H
stretching
|
Carboxylic
acid
|
1700
|
1754.72
|
C=O
stretching
|
Carboxylic
acid
|
1600-1430
|
1458.06
|
C=C
stretching
|
Aromatic
|
1310-1250
|
1306.63
|
Aromatic
C=O stretching
|
Ester
|
Table 1.2: The table of IR frequencies
for aspirin from IR spectrum.
Frequency range (cm-1)
|
Frequency
(cm-1)
|
Type of bond
|
Compound type
|
3300-2500
|
2872.66
|
O-H
stretching
|
Carboxylic
acid
|
1700
|
1754.79
|
C=O
stretching
|
Carboxylic
acid
|
1600-1430
|
1458.11
|
C=C
stretching
|
Aromatic
|
1310-1250
|
1306.33
|
Aromatic
C=O stretching
|
Ester
|
Table 1.3: The table of IR frequencies
for phenacetin from IR spectrum.
Frequency range (cm-1)
|
Frequency
(cm-1)
|
Type of bond
|
Compound type
|
3100-3000
|
2918.02
|
C-H
stretching
|
Aromatic
|
1600-1430
|
1508.76
|
C=C
stretching
|
Aromatic
|
1335-1250
|
1244.10
|
Aromatic
C-N stretching
|
Amine
|
1300-1000
|
1046.37
|
C-O
stretching
|
Ether
|
900-675
|
837.83
|
OOP C-H
stretching
|
Aromatic
|
Table 1.4: The table of IR frequencies
for caffeine from IR spectrum.
Frequency range (cm-1)
|
Frequency
(cm-1)
|
Type of bond
|
Compound type
|
1690-1640
|
1660.47
|
C=O
stretching
|
Amide
|
1640-1550
|
1545.05
|
N-H bending
|
Amide
|
1360-1080
|
1239.35
|
C-N
stretching
|
Amine
|
Table 1.5: The table of IR frequencies
for unknown A from IR spectrum.
Frequency range (cm-1)
|
Frequency
(cm-1)
|
Type of bond
|
Compound type
|
3100-3000
|
3065.93
|
C-H
stretching
|
Aromatic
|
3300-2500
|
2847.89
|
O-H
stretching
|
Carboxylic
acid
|
1700
|
1688.91
|
C=O
stretching
|
Carboxylic
acid
|
1600-1430
|
1423.95
|
C=C
stretching
|
Aromatic
|
1320-1210
|
1291.05
|
C-O
stretching
|
Carboxylic
acid
|
900-675
|
707.17
|
OOP C-H
bending
|
Aromatic
|
Table 1.6: The table of IR frequencies
for polystyrene from IR spectrum.
Frequency range (cm-1)
|
Frequency
(cm-1)
|
Type of bond
|
Compound type
|
3100-3000
|
3026.06
3060.12
3082.25
|
=C-H stretching
|
Alkene
|
3100-3000
|
3026.06
3060.12
3082.25
|
C-H stretching
|
Aromatic
|
2930
|
2923.60
|
Methylene asymmetric C-H stretching
|
Alkane
|
2000-1700
|
1744.02
1802.59
1870.69
1943.23
|
Overtone and combination bands
|
Aromatic
|
1600-1430
|
1493.01
|
C=C stretching
|
Aromatic
|
1465
|
1452.44
|
Methylene scissoring
|
Alkane
|
1680-1600
|
1601.45
|
C=C stretching
|
Alkene
|
720
|
757.13
|
Methylene rocking
|
Alkane
|
900-675
|
697.21
|
OOP C-H bending
|
Aromatic
|
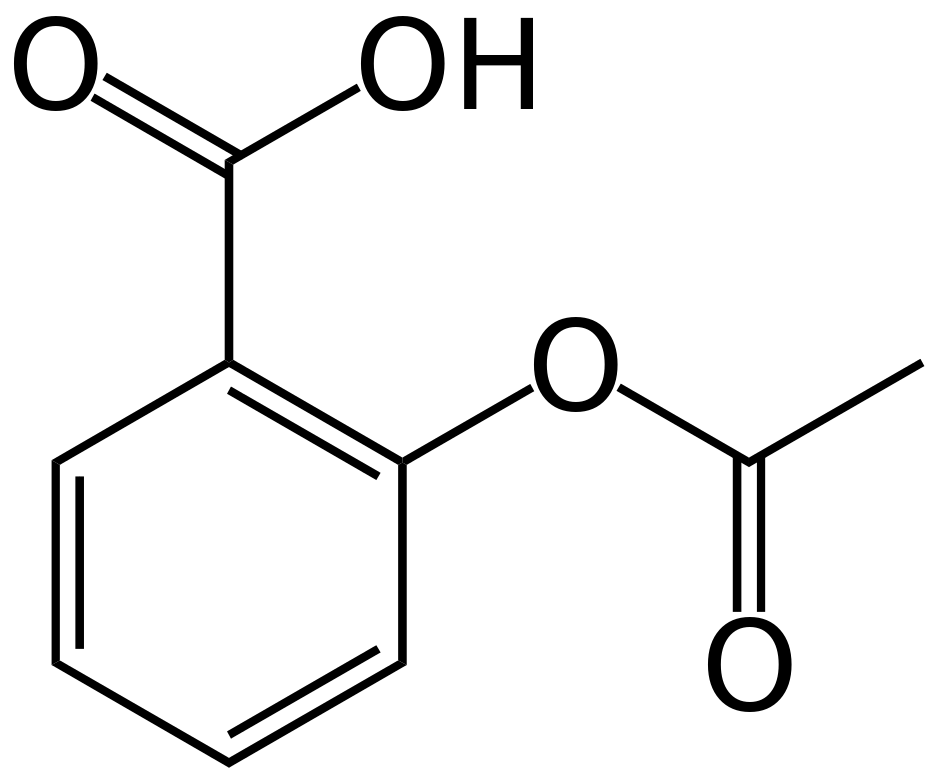
The structure of ASA:
The structure of Caffeine:
The structure of Aspirin:
The structure of unknown A = Benzoic Acid
DISCUSSION
Theoretically,
IR Spectroscopy measures the
vibrations of atoms, and based on this, it is possible to determine the
functional groups. Generally, stronger bonds and light atoms will vibrate at a
high stretching frequency (wavenumber). Chemical
bonds in different environments will absorb varying intensities and at varying
frequencies. Thus, IR spectroscopy involves collecting absorption information
and analysing it in the form of a spectrum. The frequencies at which there are
absorptions of IR radiation (peaks or signals) can be correlated directly to
bonds within the compound. Because each
interatomic bond may vibrate in several different motions which are stretching
or bending, thus individual bonds may absorb at more than one IR frequency.
Stretching absorptions usually produce stronger peaks than bending, however the
weaker bending absorptions can be useful in differentiating similar types of
bonds such as aromatic substitution. It is also important to note that symmetrical vibrations do not cause
absorption of IR radiation.
From the IR spectrum of ASA and aspirin, it shows that both
of them have about the same IR frequencies and types of bond present in the
compound. At 2917.28 cm-1, the O-H bond absorb
at this frequencies which proved the presence of carboxylic acid in the
compound. This peak display very broad, intense O-H stretching absorption in
the region of 3300-2500 cm-1. At 1754.72 cm-1, the C=O
bond absorb at this frequency where is also indicates the presence of
carboxylic acid. At frequency 1458.06, the C=C of the aromatic compound absorb
at this frequency. At 1306.63 cm-1, the aromatic C=O of the ester
absorb at this frequency with a very sharp band.
From
the IR spectrum of phenacetin, aromatic C-H stretching bands occur between
3100-3000 cm-1, where the C-H bon absorb at 2918.02 cm-1.
At 1508.76 cm-1, the C=C of the aromatic absorb at this frequency
and show less intense band. In this compound, the aromatic C-N stretching of
amine occurs at 1244.10 cm-1 with range frequencies of 1335-1250 cm-1.
The C-O bond of the ether absorb at 1046.37 cm-1 with a frequencies
range 1300-1000 cm-1. This vibrations involving oxygen atom and will
result in greater dipole moment changes than those involving carbon atoms, thus
more intense IR band is observed for ether. At 837.83 cm-1, the C-H
out-of-plane absorb at this frequency. The out-of-plane bending of a ring
hydrogen atom is strongly coupled to adjacent hydrogen atoms that will result
in the position of absorption of the OOP bands.
From
the IR spectrum of caffeine, the functional groups presences in this compound
are amide and amine. The C=O bond of the amide absorb at 1660.47 cm-1 with
frequencies range 1690-1640 cm-1, and show sharp band. While the N-H
bond of the amide absorb at 1545.05 cm-1 with frequencies range
1640-1550 cm-1. The C-N bond of the amine absorb at 1239.35 cm-1
with frequencies range 1360-1080 cm-1.
From
the IR spectrum of the unknown A, it is proved that unknown A is benzoic acid.
This is because of the presence of very broad and intense O-H stretching
absorption at a region 3300-3000 cm-1. This band may overlap with
the band from C-H stretching of the aromatic which absorb at region 3100-3000
cm-1. The sharp band at 1688.91 cm-1 indicate the
absorption of C=O of the carboxylic acid. Another sharp band at 1291.05 cm-1
indicates the absorption of C-O bond of the carboxylic acid. At 707.17 cm-1,
the OOP C-H of aromatic occurs with a very sharp band.
CONCLUSION
The functional groups in IR spectra of
standard compound aspirin, phenacetin and caffeine, the functional groups
present in aspirin-phenacetin-caffeine tablet and the functional groups present
in the unknown sample was discovered well.
REFERENCE
1. Christian, Dasgupta & Schug (2014), Analytical Chemistry 7th Edition,
p. 614
2. Mardiana Saaid, Gas Chromatography Lecture
Notes
3. Nor’ashikin S., Ruziyati T., Mardiana S.
(2012), Analytical Separation Methods Laboratory Guide (2nd edition).
4. Gas Chromatography, 4/10/2014, http://chemwiki.ucdavis.edu/Analytical_Chemistry/Instrumental_Analysis/Chromatography/Gas_Chromatography.
QUESTION
1.
The relationship between wavelength and wavenumber is wavelength
is the distance over which the shape of the wave (a cycle) repeats. While,
wavenumber is the number of full cycles in a unit distance. Their relationship
is as follows:
Wavenumber
= 1/ wavelength
2.
The conditions for IR absorption to occur are there
must be a net change in dipole moment in a molecule
as it vibrates or rotates. Dipole moment can be observed when atoms in a
molecule share electrons unequally where one atom is more electronegative than
another, resulting in that atom pulling more tightly on the shared pair of
electrons, or when one atom has a lone pair of electrons and the difference of
electronegativity vector points in the same way. As the molecule vibrates,
there is a fluctuation in its dipole moment, thus causes a field that interacts
with the electric field associated with radiation. If there is a match in
frequency of the radiation and the natural vibration of the molecule,
absorption occurs and this alters the amplitude of the molecular vibration.
This also occurs when the rotation of asymmetric molecules around their centres
results in a dipole moment change, which permits interaction with the radiation
field. Secondly, the liquid sampling cells must have extremely short pathlength
(often fractions of a millimetre), defined by the thickness of a thin polymer
film spacer.
3.
Source of IR radiation is Nernst
glower and Globar source (silicon carbide rod)
4.
A) The advantage of using KBr pellet is KBr pellet is optically transparent over the range of wavelengths
typically used in an IR analysis. While, the
disadvantage of KBr pellet is KBr is hygroscopic, thus not easy to handle.
B) The factors caused the unsatisfactory pellet are thickness of the KBr
pellet, thus light cannot penetrate through it. Secondly, less amount of sample
to be analysed compared to the amount of KBr.
5.
A) The purpose for calibration of
instrument is to ensure correct response of the instrument toward the sample to
be analysed.
B) Polystyrene is used for calibration because
it will
verify the presence of peaks seen on the IR spectra and the relative intensity
of the peaks.











No comments:
Post a Comment